We have seen the cell and gene therapy space advance at lighting speed in 2019. In January, 2019, the FDA projected 10-20 new cell and gene therapy applications each year. There are now almost 800 IND’s for cell and gene therapy programs at the start of 2020. On May 24th, 2019, the FDA approved Zolgesma, the first gene therapy for spinal muscular atrophy. Rather than offer a linear progression of incremental insights into what is likely to come, I hypothesize what may (hopefully will) happen for CRISPR in the next 12 months:
(1) Notwithstanding lingering technical issues (e.g., off-target effects and immunogenicity concerns) and public apprehension, there will be several genome editing clinical successes. This year closes with promising early clinical news from Victoria Gray, the first U.S.–based sickle-cell patient treated with a CRISPR therapy. In 2020, IND filings will increase and U.S. FDA regulators will support active recruiting for several clinical trials. I hope they will quickly generate positive results for several indications by various groups of investigators and clinicians. Both safety and efficacy for multiple indications may well be in the cards for 2020.
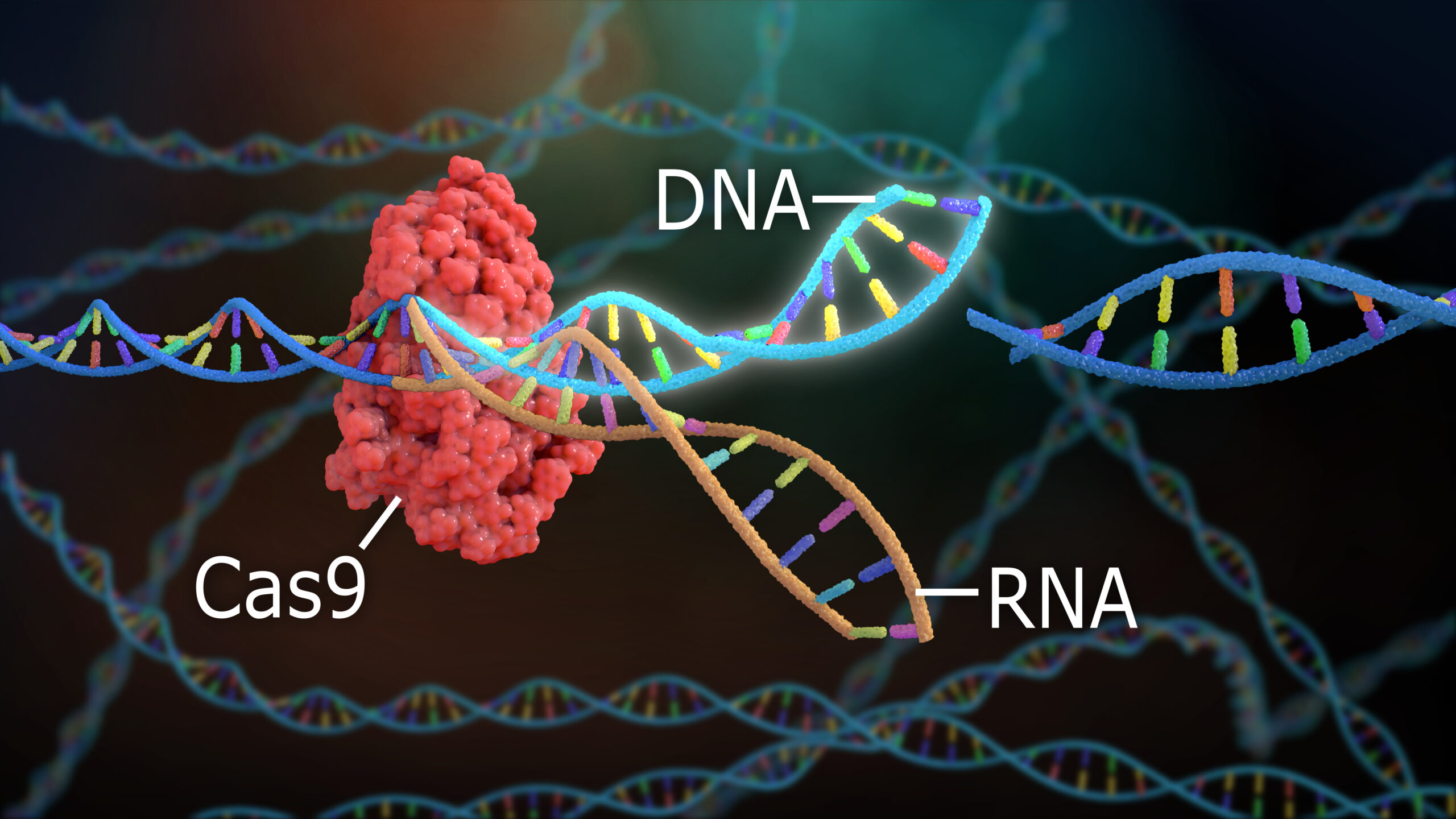
(2) Rather than continue with toolbox expansion, current CRISPR tools will be put to good use. After years of next-generation Cas mining and optimization of the Cas and fused-effector domains, we will appreciate that the current tools are polished enough for most clinical applications. With a useful Cas (Cas-9, 12, 13, et al.) toolbox, diverse applications (editing the genome, transcriptome, and epigenome) and optimized technologies (such as prime editing), currently available tools will be harnessed and implemented with greater urgency and less concerns about technology enhancement.
(3) M&A activity will increase as big pharma races to join the gene editing revolution. Investors and strategists will be intrigued by deflated stock prices given the extraordinary potential. Underperforming CRISPR stocks and underwhelming financial performance—symptoms that have affected the biotech sector as a whole—have not dampened the upside of CRISPR. The recent report of sickle-cell clinical data pushed valuations significantly higher. It would not be a surprise to see pharma competitively bid for early-stage CRISPR companies and undervalued tickers. Will we see our first CRISPR start-up acquisition in 2020?